The global 3D cell culture market, projected to reach $3.2 billion by 2028, is undergoing a fundamental paradigm shift as traditional plastic dishes and animal-derived matrices fail to capture the intricate complexity of human tissues. At the forefront of this revolution are peptide hydrogel scaffolds, synthetic biomaterials that precisely mimic the nanofibrous architecture and biochemical signals of the native extracellular matrix (ECM). These designer matrices are enabling unprecedented biological fidelity in research, with studies showing 3D cultures in peptide hydrogels exhibit gene expression profiles 60-80% closer to in vivo tissues compared to 2D plastic, while accelerating the translation of engineered tissues for regenerative medicine.
This comprehensive analysis explores how engineered peptide hydrogels are overcoming the limitations of historical models, providing a dynamically tunable, chemically defined, and ethically unambiguous platform to power the next generation of drug discovery, disease modeling, and clinical implantation.
The Limitations of Tradition: Why 2D and Natural Matrices Fall Short
Conventional cell culture methods create artificial environments that distort cell behavior, leading to poor predictive value and translational failure.
The 2D Plastic Dish Problem
How flat, rigid substrates misrepresent human biology:
- Loss of Native Morphology and Polarity: Cells flatten unnaturally, disrupting apical-basal polarization critical for function in epithelial and endothelial tissues.
- Altered Mechanotransduction: Cells sense the stiffness of glass/plastic (GPa range) rather than physiological soft tissues (0.1-30 kPa), skewing differentiation, proliferation, and migration signals.
- Compromised Cell-Cell and Cell-ECM Interactions: Limited to 2D contacts, failing to replicate the 3D network of signaling and force transmission found in vivo.
- Poor Predictivity for Drug Response: A stark 95% of drugs showing promise in 2D cancer models fail in human trials, partly due to missing 3D diffusion barriers and microenvironmental cues.
Challenges with Animal-Derived Scaffolds (Matrigel®, Collagen)
The drawbacks of using natural, but ill-defined, materials:
- Batch-to-Batch Variability: Complex composition leads to irreproducible experimental outcomes, confounding data interpretation.
- Immunogenicity and Pathogen Risk: Animal proteins can trigger immune responses, limiting therapeutic use and introducing xenogenic contamination risks.
- Limited Tunability: Fixed mechanical and biochemical properties cannot be independently adjusted to match specific tissue requirements.
- Ethical and Supply Concerns: Sourced from murine tumors, raising ethical questions and presenting scalability challenges.
“Peptide hydrogels represent the first truly designer extracellular matrix. We are no longer forced to accept the biological ‘soup’ of animal extracts or the artificial flatland of plastic. We can now program, at the molecular level, the exact physical and chemical signals we present to cells. This is the foundation for building predictive human biology in a dish.” — Dr. Sarah Chen, Director of Biomaterials, Wyss Institute.
The Science of Peptide Hydrogels: Programmable Extracellular Matrices
Peptide hydrogels are formed by the self-assembly of short synthetic peptides into water-swollen, nanofibrous networks that recapitulate key features of the native ECM.
Molecular Design and Self-Assembly Mechanisms
Engineered sequences dictate structure and function:
- Amphiphilic Peptides: Contain alternating hydrophobic and hydrophilic residues (e.g., EAEAKAKA) that form β-sheet tapes and fibrils.
- Ionic Self-Complementary Peptides: Designed with alternating positive and negative charges (e.g., RADA16-I: AcN-RADARADARADARADA-CONH₂) that form stable nanofibers in physiological salt conditions.
- Peptide Amphiphiles (PAs): Conjugate a hydrophobic alkyl tail to a peptide sequence, creating cylindrical micelles that display bioactive epitopes at high density on their surface.
- Stimuli-Responsive Assembly: Designed to gel in response to specific triggers: pH change, enzyme activity (e.g., matrix metalloproteinases), or temperature.
Tunable Material Properties for Specific Applications
Key parameters that can be independently controlled:
| Property | Typical Range | Biological Impact | How to Tune |
|---|---|---|---|
| Stiffness (Storage Modulus, G’) | 10 Pa – 10 kPa | Directs stem cell lineage (soft→neural, stiff→bone), influences cancer cell invasion. | Peptide concentration, crosslink density, fiber length. |
| Pore Size | 5 – 200 nm | Controls cell infiltration, nutrient diffusion, and neo-vascularization. | Self-assembly kinetics, peptide sequence length. |
| Degradation Rate | Days to Months | Should match the rate of new tissue deposition. Critical for scaffold resorption. | Incorporation of enzyme-cleavable linkers (e.g., MMP-sensitive sequences). |
| Bioactive Signaling | N/A | Provides specific instructions for adhesion, migration, proliferation, differentiation. | Conjugation of ECM-derived epitopes (e.g., RGD, IKVAV, YIGSR). |
Applications in Advanced 3D Cell Culture and Disease Modeling
Providing a physiologically relevant context for studying basic biology, drug efficacy, and toxicity.
Superior 3D Tumor Models for Oncology Research
Mimicking the tumor microenvironment (TME) with unprecedented accuracy:
- Gradient Formation: Peptide hydrogels allow the establishment of oxygen, nutrient, and drug gradients, driving the formation of proliferative, quiescent, and necrotic zones as in real tumors.
- Invasion and Metastasis Studies: Incorporation of MMP-cleavable sites allows real-time study of cancer cell invasion, providing a platform to test anti-metastatic drugs.
- Co-Culture Systems: Easy incorporation of cancer-associated fibroblasts (CAFs), immune cells, and endothelial cells to study complex cell-cell interactions within the TME.
- Drug Response Prediction: Cells in 3D peptide hydrogels show chemoresistance patterns (e.g., to doxorubicin) more reflective of clinical responses than 2D models, improving preclinical drug screening.
Organoid and Organ-on-a-Chip Matrices
Enabling the growth of complex, patient-derived mini-organs:
- Intestinal and Cerebral Organoids: Defined peptide hydrogels (e.g., containing laminin-derived peptides) support the growth and patterning of stem cell-derived organoids with better reproducibility than Matrigel®.
- Vascularized Tissue Models: Peptide sequences presenting angiogenic factors (e.g., VEGF-mimetic peptides) can guide the self-organization of endothelial cells into capillary-like networks within the gel.
- Mechanically Graded Systems: Creating hydrogels with spatially varying stiffness to model tissue interfaces, like the osteochondral junction (bone-cartilage).
Applications in Tissue Engineering and Regenerative Medicine
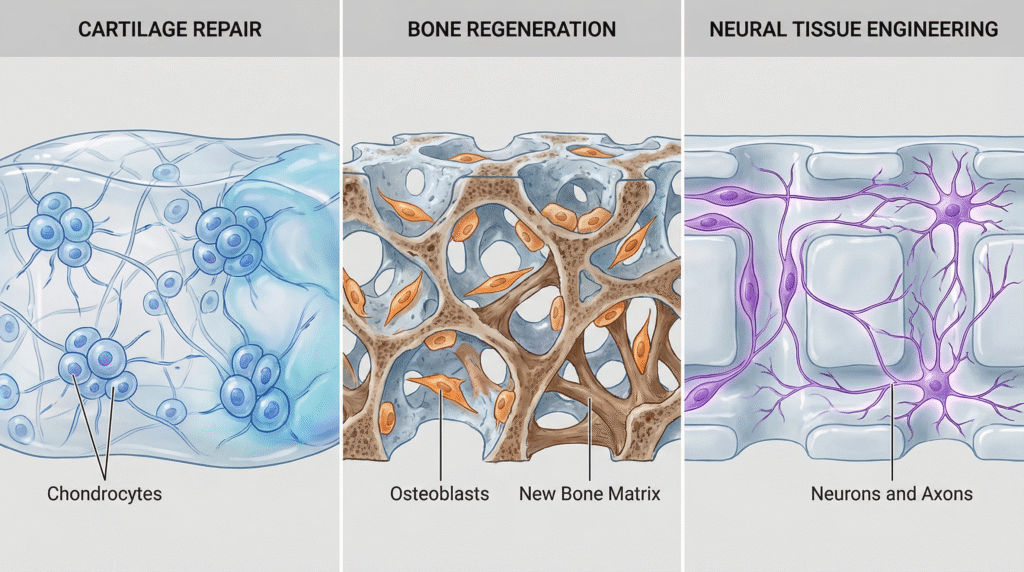
Designing instructive scaffolds to guide the body’s healing process or create fully functional implantable tissues.
Cartilage and Bone Regeneration
Addressing musculoskeletal defects:
- Cartilage Repair: Peptide hydrogels with RGD sequences and tailored stiffness (matching native cartilage, ~20 kPa) support chondrocyte encapsulation, promote glycosaminoglycan (GAG) production, and maintain a rounded cell morphology critical for function.
- Osteogenic Scaffolds: Stiffer hydrogels (≥25 kPa) functionalized with bone morphogenetic protein (BMP-2) mimetic peptides direct mesenchymal stem cell (MSC) differentiation into osteoblasts and promote mineral deposition.
- Osteochondral Defects: Bilayered or gradient hydrogels can be fabricated to simultaneously regenerate both the cartilage and underlying bone layers.
Neural Tissue Engineering
Repairing central and peripheral nervous system injuries:
- Spinal Cord Injury: Injectable peptide hydrogels (e.g., IKVAV-functionalized) provide a permissive bridge across lesion sites, promoting neurite outgrowth, reducing glial scar formation, and guiding axon regeneration in animal models.
- Peripheral Nerve Guides: Hydrogels containing laminin-derived peptides (e.g., YIGSR, IKVAV) fill nerve guidance conduits, supporting Schwann cell migration and axonal regeneration over critical gaps.
Skin and Cardiac Tissue Repair
Advancing wound healing and heart muscle regeneration:
- Diabetic Wound Healing: Hydrogels releasing angiogenic and antimicrobial peptides can be applied topically to chronic wounds, promoting vascularization and fighting infection while protecting the site.
- Myocardial Infarction: Injectable, conductive peptide hydrogels can be delivered to the infarcted heart. They provide mechanical support to prevent negative remodeling, deliver cardioprotective drugs, and improve electrical signal propagation.
Fabrication and Characterization Techniques
Advanced manufacturing and analysis are key to translating lab designs into functional products.
Advanced Manufacturing: From Simple Gels to Complex Architectures
- 3D Bioprinting: Peptide hydrogels serve as bioinks, allowing the layer-by-layer deposition of cells and materials to create complex, pre-defined tissue geometries (ears, menisci).
- Electrospinning: Creating aligned nanofiber mats from peptide-polymer blends to guide directional cell growth (e.g., for tendon/ligament repair).
- Microfluidic Fabrication: Generating microgel beads or fibers for high-throughput screening or as building blocks for modular tissue assembly.
Critical Characterization Methods
Ensuring scaffold quality and performance:
| Method | Purpose | Key Insights |
|---|---|---|
| Rheometry | Mechanical properties. | Storage/loss moduli (G’, G”), gelation kinetics, yield stress (for injectability). |
| Scanning/Transmission Electron Microscopy (SEM/TEM) | Nanofibrous structure. | Fiber diameter, alignment, network porosity. |
| In Vitro Cell Assays | Biocompatibility & bioactivity. | Cell viability, proliferation, morphology, differentiation (qPCR, immunostaining). |
| Degradation & Drug Release Profiling | Functional performance. | Mass loss over time, release kinetics of encapsulated growth factors or drugs. |
Clinical Translation, Regulatory Pathways, and Challenges
Moving peptide hydrogels from the benchtop to the bedside requires navigating a complex translational valley.
Key Challenges in Translation
- Scalable and Cost-Effective GMP Manufacturing: Transitioning from lab-scale synthesis to kilogram-scale production under strict quality control.
- Sterilization: Many peptides cannot withstand terminal sterilization (autoclaving, gamma irradiation). Aseptic processing is often required, adding complexity.
- Long-Term Stability and Shelf Life: Formulating hydrogels (often shipped as lyophilized powders or pre-gel solutions) to maintain stability for 18-24 months.
- Demonstrating Superiority: For regulatory approval, new peptide hydrogel products must demonstrate safety and effectiveness that is non-inferior or superior to existing standard-of-care treatments.
Regulatory Considerations (FDA/EMA)
Most peptide hydrogel scaffolds are regulated as Combination Products (device + biologic/drug):
- Class III Medical Device (with cells): For acellular scaffolds that primarily provide structural support, the regulatory path is that of a medical device, but often a PMA (Pre-Market Approval) is required due to the high risk.
- Biologic or ATMP (Advanced Therapy Medicinal Product): If the scaffold contains cells or is designed to actively recruit/guide host cells via significant biological action, it is regulated as a biologic or an ATMP in the EU, requiring a BLA (Biologics License Application) or MAA (Marketing Authorisation Application).
- Chemistry, Manufacturing, and Controls (CMC): Extensive data is required on peptide synthesis, purification, characterization, hydrogel formulation, and consistency of the final product.
Future Trends and Innovations
The field is rapidly evolving towards intelligent, personalized, and multifunctional systems.
Next-Generation “Smart” Hydrogels
- 4D Hydrogels: Scaffolds that change shape or properties over time in response to internal biological cues (e.g., progressively stiffen as new tissue forms).
- Immunomodulatory Scaffolds: Peptides designed to actively modulate the host immune response—promoting regenerative M2 macrophages while suppressing inflammatory M1 macrophages—to enhance integration and repair.
- Conductive and Piezoelectric Hydrogels: Incorporating conductive peptides or polymers to create scaffolds that deliver electrical stimuli for neural and cardiac tissue engineering.
Convergence with Digital Technologies
- Bioprinting with AI-Driven Design: Using machine learning to optimize peptide sequences and scaffold architectures for specific tissue functions, then directly printing them.
- Personalized Medicine: Using patient-derived cells to screen their response in 3D peptide hydrogel models, informing drug selection or customizing the design of an implantable scaffold.
FAQs: Peptide Hydrogel Scaffolds
Q: How do peptide hydrogels compare to more commonly used natural hydrogels like collagen or fibrin for 3D cell culture?
A: Peptide hydrogels offer distinct advantages in control, consistency, and customization. While collagen and fibrin are excellent natural ECM mimics, their properties (stiffness, degradation rate, adhesive ligand density) are fixed and variable between batches. Peptide hydrogels are synthetic and defined: you can precisely engineer the stiffness, pore size, and degradation profile independently. You can incorporate specific bioactive signals (e.g., one exact laminin epitope) without the “biological noise” of thousands of other proteins present in collagen. This leads to vastly superior experimental reproducibility.
The trade-off is that designing a fully functional peptide hydrogel that matches the complexity of natural ECM can be challenging; it requires a deep understanding of which specific signals are necessary for the cells of interest.
Q: What are the biggest hurdles to using peptide hydrogels for large tissue defect repair (e.g., a critical-sized bone defect)?
A: The primary hurdles are mechanical strength, vascularization, and scalability. While peptide hydrogels excel at mimicking soft tissues, they are generally weak and cannot immediately bear the load required for weight-bearing bones. Strategies to overcome this include creating composite materials with stiff polymers or bioceramics. Secondly, cells in thick implants (>~200 µm) will die without a blood supply. Promoting rapid vascularization into the scaffold remains a major challenge, addressed by incorporating angiogenic peptides or pre-forming channels.
Finally, manufacturing a large, sterile, defect-shaped scaffold with homogeneous cell distribution and consistent properties at a clinically relevant scale is a significant engineering and cost challenge.
Q: For a research lab new to 3D culture, is it feasible to start using peptide hydrogels, or are they too complex?
A: It is absolutely feasible and increasingly common. The barrier to entry has lowered significantly. Several companies now sell commercial, off-the-shelf peptide hydrogel kits (e.g., Puramatrix™, HydroMatrix™) that are simple to use: they are provided as a peptide solution that gels upon addition of cell culture media or a buffer.
These provide a well-defined, xeno-free alternative to Matrigel® for basic 3D encapsulation studies. For labs wanting to explore custom designs, many academic papers provide detailed protocols for common self-assembling peptides like RADA16-I. The key is to start with a commercially available or well-published system before attempting de novo peptide design.
Core Takeaways
- From Mimic to Design: Peptide hydrogels move beyond mimicking the ECM to actively designing it, providing an engineered, reproducible, and tunable 3D microenvironment for cells.
- Revolutionizing Preclinical Models: In drug discovery and disease modeling, 3D culture within peptide hydrogels yields data that is dramatically more predictive of human physiology than traditional 2D plastic, potentially reducing late-stage drug failures.
- Precision Tissue Engineering: The independent tunability of mechanics, biochemistry, and structure allows the creation of “instructive” scaffolds that can guide stem cell fate and tissue regeneration for specific clinical applications.
- The Clinical Translation Imperative: Success requires navigating the complex path of scalable GMP manufacturing, rigorous characterization, and strategic regulatory planning, treating the hydrogel as a critical therapeutic component, not just a passive carrier.
- The Future is Dynamic and Intelligent: Next-generation scaffolds will be responsive, immunomodulatory, and digitally designed, moving from static structures to dynamic partners in the healing and modeling process.
Conclusion: Building the Future of Biology and Medicine from the Bottom Up
Peptide hydrogel scaffolds are more than a new laboratory material; they represent a foundational technology for a new era in biological science and medicine. By providing a synthetic yet deeply biological context, they bridge the chasm between simplified cell cultures and complex living organisms. In research, they are unlocking a more authentic understanding of development, disease, and drug action. In medicine, they offer a pathway to rationally engineered tissues that can repair, restore, and regenerate. The journey from a vial of clear peptide solution to a functional, living tissue is one of the most compelling narratives in modern bioengineering.
The convergence of peptide chemistry, materials science, cell biology, and advanced manufacturing is accelerating this field from promise to reality. As designs become more sophisticated and clinical validation grows, peptide hydrogels are poised to underpin transformative advances, from personalized cancer avatars that predict treatment success to off-the-shelf implants that heal devastating injuries. The future of understanding and rebuilding the human body is being built, one nanofiber at a time, within these programmable aqueous networks.
Disclaimer
This article contains information, data, and references that have been sourced from various publicly available resources on the internet. The purpose of this article is to provide educational and informational content. All trademarks, registered trademarks, product names, company names, or logos mentioned within this article are the property of their respective owners. The use of these names and logos is for identification purposes only and does not imply any endorsement or affiliation with the original holders of such marks. The author and publisher have made every effort to ensure the accuracy and reliability of the information provided.
However, no warranty or guarantee is given that the information is correct, complete, or up-to-date. The views expressed in this article are those of the author and do not necessarily reflect the views of any third-party sources cited.




