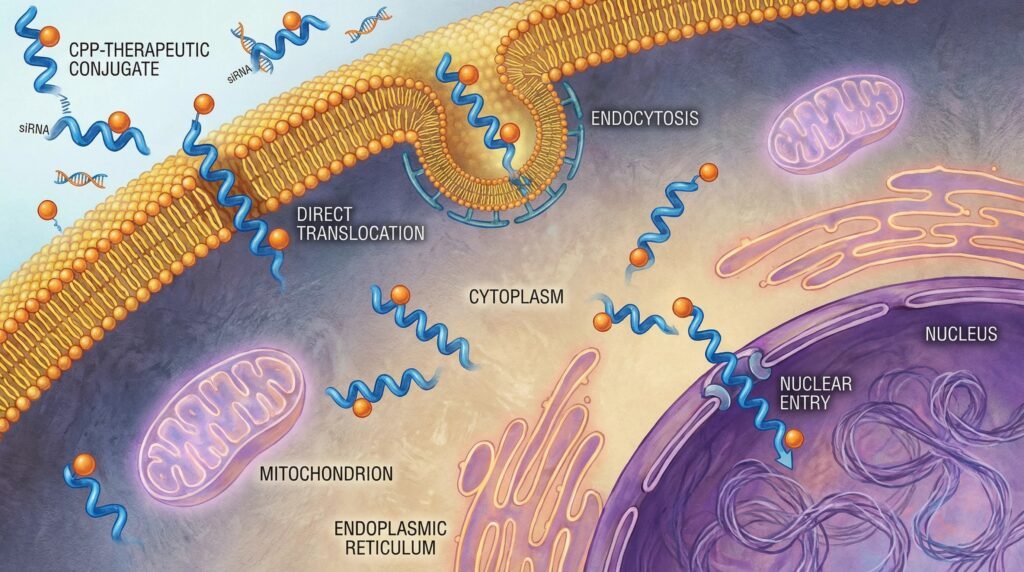
The biological cell membrane, a highly selective barrier evolved over billions of years, stands as the final and most formidable frontier for a vast array of modern therapeutics. While small-molecule drugs can often diffuse across, macromolecular agents—proteins, antibodies, nucleic acids (siRNA, mRNA, ASO), and even many peptides—are effectively barred from reaching their intracellular targets. This delivery failure is a primary reason for the high attrition rate of promising drug candidates. Cell-penetrating peptides (CPPs), also known as protein transduction domains, have emerged as a powerful biological solution to this physical problem.
By covalently or non-covalently conjugating these short, cationic, or amphipathic peptides to therapeutic cargo, scientists are creating “Trojan horse” molecules capable of ferrying impermeable payloads directly into the cellular cytoplasm and nucleus.
This article explores the cutting-edge science, clinical progress, and strategic development of peptide-cell penetrating conjugates, detailing how this technology is unlocking new therapeutic modalities for cancer, genetic disorders, and undruggable intracellular targets.
The Intracellular Delivery Challenge: A Barrier to Drugging the Undruggable
The inability to effectively deliver functional molecules inside cells limits the scope of modern medicine.
The Limitations of Conventional Therapeutics
- Macromolecular Drugs: Biologics like antibodies, enzymes, and large peptides cannot cross the lipid bilayer, restricting them to extracellular or cell-surface targets.
- Nucleic Acid Therapies: RNA-based drugs (siRNA, mRNA, ASO) and gene editing tools (CRISPR-Cas) are highly charged and large, making them impermeable and susceptible to degradation in endosomes.
- Poor Cellular Uptake: Many anticancer peptides or protein-based degraders (PROTACs) have intracellular targets but lack an efficient mechanism to enter cells.
The Endosomal Trap
Even when internalized, most large molecules are trafficked to endosomes and ultimately degraded in lysosomes, never reaching the cytosol—a fate known as the “endosomal escape” problem.
“Cell-penetrating peptides are the master keys to the cellular kingdom. They don’t just knock on the door; they provide a blueprint for engineering a delivery vehicle that can carry virtually any therapeutic cargo across the membrane and, critically, ensure it escapes the cellular recycling system to perform its function.” — Dr. Sarah Lin, Director of Drug Delivery, Novapeutics Institute.
Mechanisms of Cellular Entry: How CPPs Cross the Barrier
CPPs facilitate uptake through complex, often cargo- and context-dependent mechanisms.
| Primary Mechanism | Description | Example CPPs | Implications for Delivery |
|---|---|---|---|
| Direct Translocation | Energy-independent, rapid movement across the membrane via transient pore formation or inverted micelle structures. | Penetratin, pVEC | Avoids endosomal trapping but can be less efficient for large cargos and more sensitive to membrane composition. |
| Endocytic Uptake | Energy-dependent entry via various pathways: macropinocytosis, clathrin-mediated, caveolin-mediated endocytosis. | TAT, Transportan | High efficiency for large cargos but requires functional endosomal escape mechanisms to avoid degradation. |
| Hybrid Mechanisms | Initial electrostatic interaction with membrane components followed by endocytosis or translocation. | Most CPPs in practice | The dominant real-world mechanism, influenced by cargo, concentration, and cell type. |
Engineering the Conjugate: Linkers, Cargos, and Strategies
The success of a CPP-based therapy depends on the intelligent design of the conjugate itself.
Choice of Cell-Penetrating Peptide
CPPs are classified by their origin and properties:
- Protein-Derived: TAT (from HIV-1), Penetratin (from Drosophila Antennapedia). Well-studied but can have immunogenicity concerns.
- Chimeric/Synthetic: Transportan, MPG. Engineered for optimal properties like stability, uptake, and reduced toxicity.
- Amphipathic: Contain alternating hydrophobic and hydrophilic residues. Effective at membrane interaction and endosomal escape.
- Cationic: Rich in arginine and lysine (e.g., polyarginine). Rely on electrostatic interaction with negatively charged membrane components like heparan sulfate proteoglycans.
Conjugation Strategies and Linker Chemistry
How the CPP is attached to its cargo is critical for stability, release, and activity.
| Conjugation Type | Method | Best For | Considerations |
|---|---|---|---|
| Covalent | Stable chemical bond (amide, disulfide, thioether) between CPP and cargo. | Peptides, proteins, small molecules. | Stable in circulation. May require cleavable linker (see below) for cargo activity. |
| Non-Covalent | Electrostatic or hydrophobic complexation (e.g., CPP + nucleic acid). | Nucleic acids (siRNA, plasmid DNA). | Simpler formulation but can be less stable in vivo, with variable cargo release. |
| Cleavable Linkers | Disulfide bonds (reduced in cytosol) or protease-sensitive sequences (e.g., cathepsin B site). | Any cargo requiring intracellular release. | Enables cargo release from CPP after entry, restoring its native function. |
Therapeutic Cargos in Development
CPPs are versatile, enabling the delivery of diverse agents:
- Nucleic Acids: The most advanced area. CPP-siRNA conjugates for gene silencing; CPP-complexed mRNA for protein replacement.
- Proteins and Peptides: Delivery of tumor suppressor proteins, antibodies, or therapeutic peptides (e.g., for cancer, metabolic disease).
- Small Molecules: Enhancing the cellular uptake and efficacy of cytotoxic drugs or imaging agents.
- Nanoparticles: CPPs can be coated onto lipid or polymer nanoparticles to enhance their cellular targeting and uptake.
Clinical Translation: Status, Candidates, and Challenges
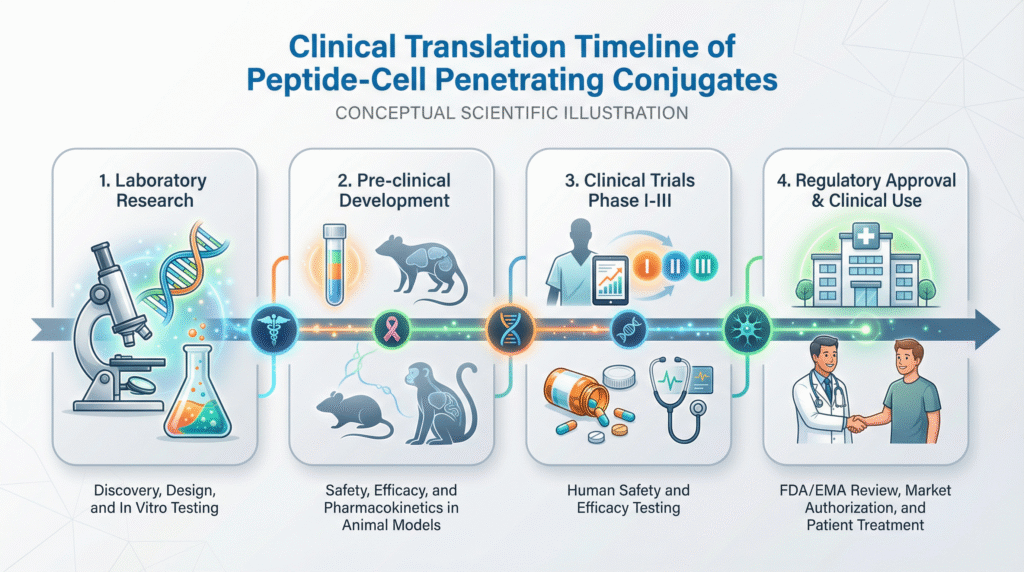
The field has moved from proof-of-concept to a growing clinical pipeline, though significant hurdles remain.
Clinical and Late-Stage Candidates
| Candidate / Platform | CPP / Strategy | Cargo / Indication | Development Status |
|---|---|---|---|
| P28 (Phase 1) | p28 peptide (derived from azurin) | Cell cycle inhibitor; solid tumors. | Completed Phase I, showed safety and tumor uptake. |
| XG-102 (Brimapitide) | TAT peptide conjugate | c-Jun N-terminal kinase (JNK) inhibitor peptide; for inner ear, ocular disorders. | Advanced clinical trials for hearing loss, uveitis. |
| Verdicetursen (Phase 2) | CPP-conjugated antisense oligonucleotide (ASO) | Targets androgen receptor variant 7 (AR-V7) mRNA; metastatic castration-resistant prostate cancer. | Phase II studies completed. |
| Various CPP-siRNA Conjugates | Targeted, stabilized CPPs | Oncology, metabolic diseases. | Multiple candidates in Phase I/II. |
Key Development Challenges and Solutions
- Lack of Cell/Organ Specificity: CPPs often enter all cells. Solutions: Activatable CPPs (shielded until they reach the target), coupling with targeting ligands (antibodies, peptides).
- Endosomal Entrapment: A major bottleneck. Solutions: Use of CPPs with endosomolytic activity (e.g., containing histidine-rich fusogenic sequences), co-treatment with endosomolytic agents.
- Poor In Vivo Stability & Pharmacokinetics: CPPs and their conjugates can be rapidly cleared. Solutions: PEGylation, D-amino acid substitution, cyclization to enhance proteolytic stability.
- Immunogenicity and Toxicity: Cationic CPPs can cause membrane disruption and cytotoxicity at high doses. Solutions: Engineering less toxic sequences, dose optimization.
Future Directions and Next-Generation Platforms
The field is evolving towards smarter, more targeted, and responsive delivery systems.
Advanced Engineering and Design
- AI-Driven CPP Discovery: Machine learning models are screening vast peptide sequence spaces to identify novel CPPs with optimal properties for specific cargos and cell types.
- Stimuli-Responsive “Smart” CPPs: Conjugates that are activated only in the tumor microenvironment (by low pH, specific enzymes) to minimize off-target effects.
- CPPs for Organelle-Specific Delivery: Designing peptides that not only enter the cell but also localize to mitochondria, nucleus, or endoplasmic reticulum.
Expanding Therapeutic Horizons
- Delivery of Gene Editing Tools: CPP-based delivery of CRISPR-Cas ribonucleoproteins (RNPs) offers a transient, non-viral alternative for gene editing.
- Crossing the Blood-Brain Barrier (BBB): Engineered CPPs show promise in shuttling therapeutics into the brain, a major challenge for neurological diseases.
- Intracellular Protein Degradation: CPPs are being used to deliver molecular glues or PROTACs that target intracellular proteins for degradation.
FAQs: Peptide-Cell Penetrating Conjugates
Q: What is the main advantage of using a covalent CPP conjugate over simply mixing the CPP with the cargo?
A: The primary advantage is controlled stoichiometry and stability. A covalent conjugate ensures a defined 1:1 (or other specific) ratio of CPP to cargo molecule, which leads to more reproducible cellular uptake and pharmacological activity. In circulation, the covalent bond prevents dissociation, ensuring the cargo is delivered as part of the conjugate. Simple mixing (non-covalent complexation) can result in heterogeneous particles, premature cargo release in the bloodstream, and less predictable behavior in vivo, although it is a simpler method often used for nucleic acid delivery in research settings.
Q: Are CPP conjugates immunogenic? Could the body develop antibodies against the CPP, limiting repeated dosing?
A: Immunogenicity is a valid concern, particularly for CPPs derived from viral or foreign proteins (like TAT). The immune system can recognize these peptide sequences and generate antibodies, potentially leading to accelerated clearance upon repeat dosing or adverse reactions. The field is addressing this by designing fully synthetic, de novo CPPs with sequences not found in nature to minimize immune recognition. Additionally, techniques like PEGylation can shield the conjugate. Immunogenicity assessment is a standard part of the non-clinical and clinical safety package for any CPP conjugate therapeutic.
Q: For a research lab wanting to test a new intracellular-targeting peptide, what is a good first CPP to try for proof-of-concept conjugation?
A: For an initial proof-of-concept, TAT (GRKKRRQRRRPQ) or a simple polyarginine peptide (e.g., R9) are common and effective starting points. They are well-characterized, commercially available, and show robust uptake in many cell types. The conjugation can be done via a standard maleimide-thiol or click chemistry reaction if your peptide has a modifiable cysteine or lysine. It’s important to include proper controls: the cargo peptide alone, the CPP alone, and a scrambled CPP sequence conjugate to confirm that uptake is indeed mediated by the CPP. For therapeutic development, more advanced, engineered CPPs would be necessary.
Core Takeaways
- Breaking the Delivery Barrier: Cell-penetrating peptides provide a versatile and powerful solution to the critical challenge of intracellular delivery, enabling a new class of macromolecular and nucleic acid therapeutics.
- Mechanism-Informed Design: Successful conjugates require careful selection of the CPP (cationic, amphipathic), the conjugation strategy (covalent with cleavable linkers), and engineering to overcome endosomal entrapment.
- Growing Clinical Pipeline: CPP-conjugates, particularly for siRNA and peptide drugs, are advancing in clinical trials for oncology, genetic, and metabolic diseases, validating the translational potential of the technology.
- Specificity and Safety are Key Challenges: Next-generation platforms focus on creating targeted, activatable, and non-immunogenic CPPs to improve the therapeutic index and enable systemic administration.
- A Platform for Innovation: CPP technology is a foundational delivery platform that will continue to enable new modalities, from gene editing and intracellular protein degradation to crossing the blood-brain barrier.
Conclusion: Unlocking the Full Potential of Intracellular Therapeutics
The development of peptide-cell penetrating conjugates represents a paradigm shift in drug delivery, transforming biologically active molecules that were once confined to the extracellular space into potent intracellular agents. As our understanding of CPP mechanisms deepens and protein engineering tools become more sophisticated, the limitations of early systems—lack of specificity, endosomal trapping, stability—are being systematically addressed. The result is a new generation of smarter, more effective delivery vehicles poised to bring previously undruggable targets within therapeutic reach.
The complexity of designing an effective and manufacturable CPP conjugate cannot be understated. It requires a deep synergy between peptide chemistry, formulation science, and biology. Success hinges on the quality and reliability of the core building block: the peptide itself. Sichuan Pengting Technology Co., Ltd. provides a critical foundation for this innovation. As a professional and reliable peptide API supplier, we enable the advancement of CPP-conjugate therapeutics by providing high-purity, custom-designed cell-penetrating peptides and cargo peptides with precise modifications (e.g., for site-specific conjugation).
Our expertise in complex peptide synthesis ensures researchers and developers have access to the well-characterized, GMP-ready peptide components essential for building robust conjugates, conducting reliable preclinical studies, and ultimately scaling towards clinical and commercial manufacturing. Partnering with a trusted peptide specialist like Sichuan Pengting Technology allows innovators to focus on the biological and therapeutic challenges, confident in the quality and supply of their core peptide materials.
Disclaimer
This article contains information, data, and references that have been sourced from various publicly available resources on the internet. The purpose of this article is to provide educational and informational content. All trademarks, registered trademarks, product names, company names, or logos mentioned within this article are the property of their respective owners. The use of these names and logos is for identification purposes only and does not imply any endorsement or affiliation with the original holders of such marks. The author and publisher have made every effort to ensure the accuracy and reliability of the information provided.
However, no warranty or guarantee is given that the information is correct, complete, or up-to-date. The views expressed in this article are those of the author and do not necessarily reflect the views of any third-party sources cited.




