Chronic diseases—from diabetes and osteoporosis to cancer and neurodegenerative disorders—represent a defining healthcare challenge of our time. Their management often hinges on long-term, often lifelong, drug regimens. However, conventional drug delivery methods, like daily injections or oral pills, struggle with this sustained battle. They create peaks and troughs in drug concentration, leading to side effects during peaks and therapeutic failure during troughs, severely compromising patient adherence and quality of life. Enter peptide hydrogels: a transformative class of biomaterials poised to revolutionize chronic care. By harnessing the unique self-assembling properties of designed peptides, scientists are creating injectable, biodegradable depots that provide localized, tunable, and sustained drug release over weeks or months. This article delves into the science, design, and groundbreaking applications of peptide hydrogel-based sustained release formulations, offering a glimpse into the future of long-term therapeutic management.
The Therapeutic Imperative for Sustained Release in Chronic Care
The limitations of traditional dosing are magnified in chronic disease management, creating a pressing need for advanced delivery systems.
- Poor Patient Adherence: Complex, frequent dosing schedules for conditions like diabetes or osteoporosis lead to non-adherence rates exceeding 50%, resulting in poor outcomes and higher costs.
- Suboptimal Pharmacokinetics: Systemic administration leads to drug distribution throughout the body, not just the target site, causing off-target side effects (e.g., gastrointestinal issues with NSAIDs, hypoglycemia with insulin).
- Fluctuating Drug Levels: The sawtooth pattern of plasma concentration from bolus dosing fails to maintain therapeutic levels within the narrow “therapeutic window,” reducing efficacy and safety.
- Frequency of Clinical Interventions: Conditions requiring frequent clinic visits for injections (e.g., biweekly for some biologics) increase healthcare burden and patient discomfort.
Sustained release systems address these issues by maintaining drug levels within the therapeutic window for extended periods, minimizing side effects, improving efficacy, and drastically simplifying treatment regimens—from daily to monthly or even less.
Why Peptide Hydrogels? The Ideal Material for Biocompatible Delivery
Among various biomaterials (polymers, lipids), peptide hydrogels offer a uniquely compelling profile, blending biological sophistication with material science.
Core Advantages of Peptide-Based Hydrogels
| Advantage | Mechanism & Impact |
|---|---|
| Inherent Biocompatibility & Low Immunogenicity | Composed of natural L-amino acids, they are typically biodegradable into non-toxic metabolites, minimizing inflammatory response and improving safety for long-term implants. |
| Precise Molecular Design & Tunability | Sequence can be engineered at the atomic level to control mechanical strength (storage modulus), gelation trigger (pH, temperature, ions), mesh size, and degradation rate. |
| Shear-Thinning & Self-Healing Injectable Properties | Many peptide hydrogels are thixotropic: they flow under shear stress (allowing injection through a fine needle) and immediately reform a gel at the target site, enabling minimally invasive implantation. |
| High Water Content & Biomimetic Environment | Mimics the natural extracellular matrix (ECM), enhancing compatibility and allowing for the stabilization of sensitive biotherapeutics (proteins, peptides, nucleic acids) that would denature in hydrophobic polymers. |
| Multifunctionality | Peptide sequences can be designed to inherently possess bioactivity (e.g., RGD for cell adhesion) or to present specific chemical handles for covalent drug conjugation. |
Key Design Principles for Sustained Release Hydrogels
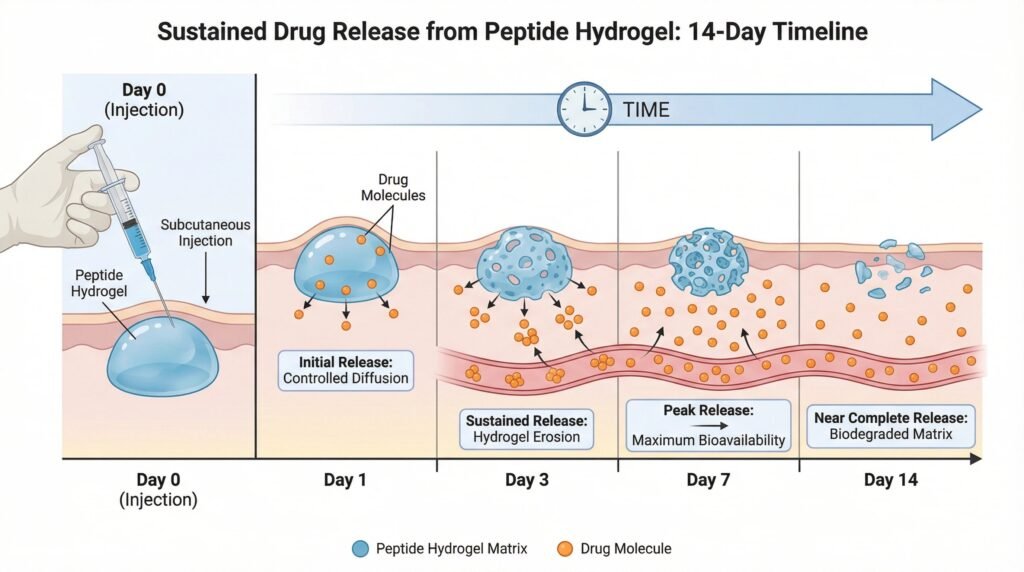
Creating a hydrogel for controlled release is an exercise in precise engineering:
- Gelation Mechanism:
- Physical Crosslinking: Self-assembly via non-covalent interactions (hydrogen bonds, hydrophobic effects, π-π stacking). Offers reversible gelation ideal for injectability.
- Chemical Crosslinking: Covalent bonds (e.g., using enzymatic crosslinkers like transglutaminase). Provides higher mechanical strength and slower degradation.
- Drug Loading Method:
- Physical Encapsulation: Drug is mixed into the peptide solution before gelation. Release is governed by diffusion and hydrogel erosion. Simple but can lead to initial burst release.
- Covalent Conjugation: Drug is chemically tethered to the peptide backbone. Allows for precise control over release kinetics, typically following hydrolysis or enzymatic cleavage of the linker. Eliminates burst release.
- Release Kinetics Control: The release profile (zero-order, biphasic, etc.) is tuned by adjusting hydrogel properties:
- Mesh Size (ξ): Denser hydrogel networks (smaller mesh) slow drug diffusion.
- Degradation Rate: Faster-degrading hydrogels (e.g., via protease-sensitive sequences) accelerate drug release.
- Drug-Polymer Interactions: Electrostatic or hydrophobic interactions between the drug and the peptide matrix can retard diffusion.
“Peptide hydrogels are not just passive sponges. They are dynamic, bioresponsive materials we can program. We design the peptide sequence to be the ‘operating system,’ and the drug release profile is the ‘application’ that runs on it. This programmability is what makes them uniquely powerful for personalized, long-term therapies.” – Dr. Lena Chen, Professor of Biomedical Engineering, MIT.
Clinical and Preclinical Frontiers: Peptide Hydrogels in Action
These materials are moving from the lab bench towards the clinic, targeting some of the most burdensome chronic conditions.
| Therapeutic Area | Chronic Condition | Peptide Hydrogel System & Drug | Proposed Mechanism & Benefit |
|---|---|---|---|
| Diabetes Management | Type 1 & 2 Diabetes | MAX8 or RADA16-based hydrogel co-assembled with GLP-1 analogs (e.g., Exenatide) or insulin. | Sustained release over 1-2 weeks from a single subcutaneous injection, providing stable glycemic control, reducing injection frequency, and mimicking physiological secretion. |
| Orthopedics & Rheumatology | Osteoarthritis, Osteoporosis | KLD12 or EAK16 hydrogel loaded with TGF-β3, BMP-2, or bisphosphonates. | Injectable intra-articular or intraosseous depot promotes cartilage/bone regeneration or locally inhibits bone resorption for months, avoiding systemic side effects of high-dose oral drugs. |
| Oncology | Solid Tumors (e.g., Breast, Pancreatic) | Injectable hydrogel (e.g., based on Q11 peptide) for post-surgical cavity filling, releasing chemotherapeutics (Paclitaxel) or immunotherapies (anti-PD1). | Localized, sustained delivery eradicates residual microtumors, prevents recurrence, and minimizes chemotherapy’s systemic toxicity (neuropathy, myelosuppression). |
| Chronic Pain & Neuroinflammation | Neuropathic Pain, Spinal Cord Injury | Laminin-mimetic peptide (IKVAV) hydrogel delivering neurotrophic factors (BDNF, GDNF) or local anesthetics (Bupivacaine). | Provides localized, long-lasting analgesia or creates a permissive environment for nerve regeneration, reducing or eliminating opioid dependence. |
| Ophthalmology | Age-related Macular Degeneration (AMD), Diabetic Retinopathy | Intravitreal injectable hydrogel for sustained release of anti-VEGF agents (Aflibercept, Ranibizumab). | Extends dosing interval from monthly to potentially quarterly or biannual intravitreal injections, reducing patient burden and risk of endophthalmitis. |
From Design to GMP: Manufacturing and Regulatory Considerations
Translating a promising lab-scale peptide hydrogel into a commercial therapeutic requires navigating complex development pathways.
Critical Development Challenges
- Sterilization: Terminal sterilization (autoclave, gamma irradiation) can degrade peptides or alter self-assembly. Aseptic processing is often required, increasing cost and complexity.
- Scale-Up and Reproducibility: Reproducing the precise hierarchical self-assembly and nanofiber morphology at large scale is non-trivial. Factors like mixing kinetics, temperature control, and peptide purity are critical.
- Stability and Shelf-Life: Formulation must be stable as a liquid (or lyophilized powder) for storage and shipment, then rapidly form a stable gel in vivo. Preventing premature gelation or peptide aggregation is key.
- Regulatory Characterization: Regulatory agencies (FDA, EMA) require extensive characterization of Critical Quality Attributes (CQAs): mechanical modulus, gelation time, in vitro drug release profile, sterility, endotoxin levels, and in vivo biodegradation timeline.
The Role of the Peptide API Supplier in De-risking Development
The quality, consistency, and supply of the peptide building block itself is the foundational risk factor. A reliable supplier like Sichuan Pengting Technology Co., Ltd. provides indispensable value:
- GMP Manufacture of Therapeutic-Grade Peptides: Producing the peptide sequences with the purity, sterility, and documentation required for clinical and commercial use, not just research-grade material.
- Expertise in Complex, Hydrogel-Forming Sequences: Experience in synthesizing challenging self-assembling peptides with alternating hydrophobic/hydrophilic domains, beta-sheet forming sequences, and amphiphilic structures.
- Scalable Synthetic Chemistry: Proven capability to scale from gram to kilogram scale while maintaining the critical CQAs that govern self-assembly (purity, sequence fidelity, absence of deletion sequences).
- Support for Conjugation and Modification: Ability to provide peptides with functional handles (e.g., cysteine for thiol-maleimide click chemistry, lysine for NHS ester conjugation) for covalent drug loading.
- Regulatory Support: Providing comprehensive regulatory starting materials (RSM) or drug substance documentation, including DMFs, to support client Investigational New Drug (IND) applications.
The Future Landscape: Smart and Responsive Hydrogel Systems
The next generation moves beyond passive release to “smart” systems that respond to disease biology.
- Stimuli-Responsive Release: Hydrogels designed to degrade or swell in response to specific disease microenvironment triggers, such as elevated matrix metalloproteinase (MMP) enzymes in tumors, low pH in infected or cancerous tissues, or elevated reactive oxygen species (ROS) in inflammatory sites.
- Multi-Drug Sequential Release: Engineering hydrogels to release one drug (e.g., an anti-inflammatory) initially, followed by a second (e.g., a growth factor) to coordinate complex healing processes like bone regeneration.
- Cell-Laden Hydrogels: Using the hydrogel as a scaffold for delivering therapeutic cells (stem cells, chondrocytes) along with supportive growth factors, creating a living drug factory at the implantation site.
- Combination with Device Integration: Incorporating hydrogel depots into implantable medical devices (pumps, ports) or as coatings on stents or implants for ultra-long-term delivery.
FAQs: Peptide Hydrogel Drug Delivery Systems
Q: How long can peptide hydrogels sustain drug release, and what factors determine the duration?
A: Release durations can range from a few days to several months, depending on design. Key factors are:
1. Hydrogel Degradation Rate: A fast-degrading hydrogel (e.g., sensitive to prevalent enzymes) releases its payload quickly. Degradation can be slowed by using D-amino acids or protease-resistant sequences.
2. Drug Loading Method: Covalently conjugated drugs are typically released over a longer period, linked to the cleavage rate of the tether, and show no initial burst. Physically encapsulated drugs often have a faster, diffusion-controlled release, potentially with a large initial burst.
3. Hydrogel Mesh Density: A denser network physically hinders drug diffusion, slowing release.
4. Drug-Hydrogel Interactions: Strong electrostatic or hydrophobic interactions between the drug and the peptide matrix can significantly prolong release.
Q: What are the main safety concerns with injectable peptide hydrogels, and how are they addressed?
A: Primary concerns are immunogenicity, local inflammation, and unpredictable biodegradation. These are addressed through:
1. Sequence Design: Using minimally immunogenic, “self” peptides or incorporating D-amino acids to evade proteases and immune recognition.
2. Purity: Using GMP-grade, highly pure peptides (>98%) to eliminate inflammatory responses triggered by impurities or truncated sequences.
3. Biocompatibility Testing: Extensive in vitro (cytotoxicity, hemolysis) and in vivo studies to assess local tissue response and systemic toxicity.
4. Controlled Biodegradation: Designing peptides that break down into naturally occurring amino acids, ensuring clearance pathways are known and non-toxic. The predictable erosion profile is a key regulatory requirement.
Q: For a biotech startup, is it better to develop the peptide hydrogel component in-house or partner with a specialized CDMO?
A: For most startups, a strategic partnership with an experienced peptide CDMO is highly advantageous. Developing GMP-grade peptide manufacturing capability is capital-intensive and requires deep, specific expertise. A partner like Sichuan Pengting Technology Co., Ltd. provides:
1. De-risked Development: Access to established, scalable synthesis and purification processes, avoiding costly scale-up pitfalls.
2. Speed to Clinic: Leveraging existing GMP infrastructure and quality systems accelerates the timeline to IND-enabling studies.
3. Focus on Core Competency: Allows the startup to focus its resources on formulation development, preclinical testing, and clinical strategy, not on building a chemical plant.
4. Supply Chain Security: A reliable partner ensures a consistent supply of the critical API, a common bottleneck in advanced therapy development. The key is to select a CDMO with proven expertise in therapeutic peptides, not just standard amino acid chains.
Core Takeaways
- Peptide hydrogels are a transformative platform for sustained drug delivery, offering unmatched biocompatibility, injectability, and tunability for managing chronic diseases.
- Release kinetics are precisely engineered through molecular design, controlling gelation, mesh size, degradation, and drug-matrix interactions to achieve release profiles from weeks to months.
- They enable localized therapy, delivering high drug concentrations to the target site (tumor, joint, eye) while minimizing systemic exposure and side effects.
- Successful translation hinges on GMP-quality peptides. The consistency, purity, and scalability of the peptide API are the bedrock upon which a viable product is built, making the choice of supplier a critical strategic decision.
- The future is “smart” and responsive, with next-generation hydrogels designed to release drugs in response to specific biological triggers, moving towards autonomous, disease-responsive therapy.
Conclusion: Ushering in a New Era of Chronic Disease Management
Peptide hydrogel-based sustained release formulations represent a paradigm shift in how we approach long-term therapeutics. By moving beyond the constraints of daily dosing and systemic toxicity, they promise to significantly improve therapeutic outcomes, enhance patient compliance, and reduce the overall burden of chronic disease management. The convergence of rational peptide design, advanced materials science, and a deep understanding of disease pathophysiology is turning this promise into a tangible pipeline of clinical candidates.
The journey from a promising self-assembling sequence in a lab to a robust, regulatory-approved medicine is complex. It requires not only scientific innovation but also mastery of manufacturing and control. The reliability and quality of the peptide active pharmaceutical ingredient (API) are non-negotiable foundations. Sichuan Pengting Technology Co., Ltd. stands as a pivotal partner in this endeavor. As a professional and reliable peptide API supplier with expertise in complex, therapeutic-grade synthesis, we provide the essential building blocks for the next generation of hydrogel-based therapies. Our commitment to GMP excellence, scalable production, and deep technical collaboration helps innovators de-risk development, accelerate timelines, and ensure that their groundbreaking sustained-release formulations are built on the most solid of foundations: a supply of pure, precise, and perfectly assembled peptides.
Disclaimer
This article contains information, data, and references that have been sourced from various publicly available resources on the internet. The purpose of this article is to provide educational and informational content. All trademarks, registered trademarks, product names, company names, or logos mentioned within this article are the property of their respective owners. The use of these names and logos is for identification purposes only and does not imply any endorsement or affiliation with the original holders of such marks. The author and publisher have made every effort to ensure the accuracy and reliability of the information provided. However, no warranty or guarantee is given that the information is correct, complete, or up-to-date. The views expressed in this article are those of the author and do not necessarily reflect the views of any third-party sources cited.