
The global peptide therapeutics market, on a trajectory to surpass $75 billion, is confronting a pivotal question from regulators, investors, and patients: what is the true origin and journey of these life-saving molecules? In an era defined by stringent ESG (Environmental, Social, and Governance) mandates, where over $30 trillion in assets are managed under sustainability principles, opaque supply chains are no longer a competitive disadvantage—they are an existential risk. For peptide APIs, often synthesized from globally sourced amino acids and complex intermediates, the lack of visibility beyond the first-tier manufacturer creates vulnerabilities to adulteration, unethical labor practices, and environmental harm.
This comprehensive guide explores the imperative for peptide supply chain transparency, detailing the cutting-edge traceability systems and robust verification frameworks that enable companies to ensure ethical sourcing, mitigate regulatory and reputational risk, and build the resilient, trustworthy supply networks demanded by the future of medicine.
The Imperative for Transparency: Beyond Compliance to Core Value
Transparency is evolving from a passive disclosure to an active, strategic capability that protects patient safety, ensures business continuity, and fulfills corporate citizenship.
The Tangible Risks of an Opaque Supply Chain
Operating without visibility introduces multiple, compounding threats:
- Quality and Safety Risks: Inability to trace raw materials (e.g., amino acids) to their source increases the risk of adulteration, contamination, or substitution with non-GMP materials, directly jeopardizing drug efficacy and patient safety.
- Regulatory and Legal Exposure: Laws like the U.S. Uyghur Forced Labor Prevention Act (UFLPA) and the EU’s forthcoming Corporate Sustainability Due Diligence Directive (CSDDD) impose strict liability for human rights abuses in supply chains, with severe penalties including import bans.
- Reputational Catastrophe: Discovery of unethical practices at a sub-tier supplier can trigger consumer boycotts, investor divestment, and irreversible brand damage, regardless of the sponsor’s direct knowledge.
- Operational Disruption: A quality or ethical incident at an unknown lower-tier supplier can halt production for months while alternative sources are qualified.
The Business Value of a Transparent Chain
Proactive transparency delivers measurable advantages:
- Enhanced Resilience: Mapping the multi-tier supply chain identifies single points of failure and enables proactive risk mitigation and diversification.
- Competitive Differentiation: A verifiably ethical and transparent supply chain is a powerful differentiator for tenders with large pharma companies and healthcare systems with net-zero and ethical sourcing commitments.
- Access to Green Capital: ESG-focused investors and lenders increasingly require detailed supply chain disclosures as a condition for financing.
- Accelerated Problem-Solving: When a deviation occurs, a digital traceability system can pinpoint the affected batches and their location in hours, not weeks, minimizing recall scope and cost.
“In the peptide world, we are not just synthesizing molecules; we are curating a narrative of origin, integrity, and responsibility. The patient’s trust begins not at the pharmacy counter, but at the mine where a metal catalyst is sourced or the farm where a fermentation feedstock is grown. Traceability is the technology that lets us tell that story truthfully and defend it rigorously.” — Marcus Thorne, Chief Supply Chain Officer, Global Biologics Logistics.
The Technology Arsenal: Modern Traceability Systems
Achieving end-to-end visibility requires a combination of digital identifiers, data capture points, and secure sharing platforms.
Digital Identifiers and Serialization
The foundational layer for tracking individual items:
- Unique Batch/Lot Identifiers: Extending beyond the final API batch to include unique identifiers for all input materials (amino acids, resins, solvents).
- 2D Barcodes (QR Codes, DataMatrix): Attached to containers, enabling quick scanning to retrieve a digital record of the item’s journey and attributes.
- Radio-Frequency Identification (RFID): For pallets and large containers, allowing automated, non-line-of-sight tracking through warehouses and ports.
Blockchain and Distributed Ledger Technology (DLT)
Providing an immutable, shared record of transactions across a permissioned network:
| Feature | Benefit for Peptide Supply Chains |
|---|---|
| Immutability | Once a transaction (e.g., “Batch A of Fmoc-Leucine shipped from Supplier X to CMO Y”) is recorded, it cannot be altered, providing a single source of truth. |
| Decentralization | No single entity controls the data; all permissioned participants (supplier, CMO, sponsor) have the same synchronized view, reducing disputes. |
| Smart Contracts | Automated execution of agreements. Example: A certificate of analysis (CoA) is automatically released to the buyer upon confirmation of receipt and temperature compliance. |
| Provenance Tracking | Creates an unbroken digital thread from raw material origin to finished API, ideal for proving ethical sourcing claims. |
Internet of Things (IoT) and Integration Platforms
Capturing and communicating real-time physical data:
- IoT Sensors: Monitor and record temperature, humidity, and shock during transportation, linking this environmental data to the specific batch’s digital record.
- ERP and QMS Integration: Traceability systems must pull data from enterprise resource planning (ERP) and quality management systems (QMS) to automatically record batch manufacturing, test results, and shipment events.
- Supplier Portals: Cloud-based platforms where suppliers can directly upload their own certificates, audit reports, and sourcing declarations, ensuring data authenticity and reducing manual entry.
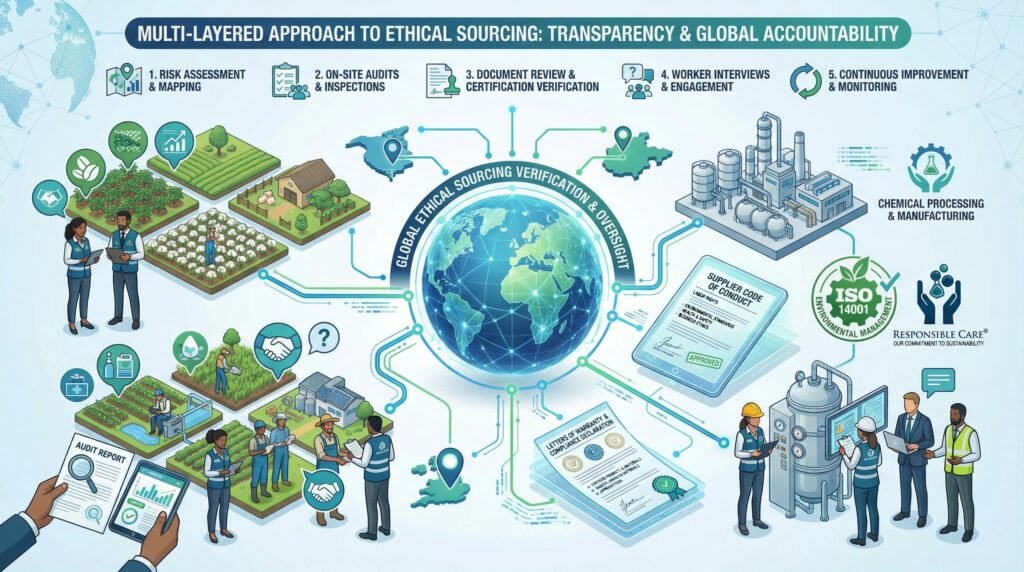
Verifying Ethical Sourcing: A Multi-Layered Framework
Traceability provides the “where,” but ethical verification answers the “how.” This requires a combination of audits, certifications, and technological proof.
1. Supplier Due Diligence and Risk Assessment
Proactively mapping and evaluating the supply network:
- Multi-Tier Mapping: Using questionnaires and software tools to identify suppliers at Tier 2, 3, and beyond, particularly for high-risk commodities (e.g., solvents, metals, amino acids from regions with known labor or environmental risks).
- Geopolitical and ESG Risk Scoring: Applying risk ratings to suppliers based on their location, commodity type, and known sectoral challenges.
- Ownership and Corporate Structure Analysis: Investigating ultimate beneficial ownership to screen for connections to sanctioned entities or high-risk jurisdictions.
2. Audits, Certifications, and Third-Party Verification
Independent validation of ethical claims:
| Tool | Focus Area | Relevance to Peptide Inputs |
|---|---|---|
| Social Audits (SMETA, SA8000) | Labor practices, health & safety, working hours. | For manufacturers of chemical intermediates, amino acids, and packaging materials. |
| Environmental Certifications (ISO 14001) | Environmental management systems. | Verifies suppliers’ commitment to reducing pollution, waste, and water use. |
| Responsible Care® | Chemical industry safety, health, environmental, and security practices. | A key certification for chemical and solvent suppliers. |
| Third-Party Verification Services | Independent on-site assessments of specific ethical claims (e.g., no forced labor, sustainable farming). | For high-risk raw materials, providing an extra layer of assurance beyond self-reporting. |
3. Documentary Evidence and Chain of Custody
Collecting and verifying the paper (and digital) trail:
- Supplier Code of Conduct: A mandatory, signed agreement requiring suppliers to adhere to defined labor, ethical, and environmental standards, with flow-down obligations to their own suppliers.
- Letters of Warranty: Formal statements from suppliers warranting that materials were produced in compliance with specific laws (e.g., UFLPA).
- Chain of Custody (CoC) Certificates: Documents that track the physical custody of a material from origin through each handler. For peptides, this can be digitized and linked to the batch’s blockchain record.
Implementation Roadmap: Building a Transparent Peptide Supply Chain
Transparency is a journey, not a destination. A phased, pragmatic approach is essential for success.
Phase 1: Assess and Prioritize (Months 0-3)
- Conduct a Supply Chain Mapping Exercise: Identify all direct (Tier 1) suppliers of peptide APIs and key raw materials.
- Perform a Risk Assessment: Rank suppliers based on spend, criticality, geographic risk, and commodity type to focus efforts.
- Define Policy and Goals: Establish a corporate Ethical Sourcing Policy and set clear, measurable transparency goals (e.g., “Map 100% of Tier 1 suppliers and 50% of Tier 2 by end of Year 2”).
Phase 2: Engage and Digitize Core Network (Months 4-18)
- Onboard Strategic Suppliers: Engage top-priority suppliers. Require them to complete detailed profiles, sign the Code of Conduct, and begin sharing key data (CoAs, audit reports) via a portal.
- Pilot a Track-and-Trace Program: Select one high-value peptide API and implement a digital traceability pilot from your CMO back to the key starting materials. Use simple 2D barcodes and a cloud-based system.
- Conduct Focused Audits: Perform or request audits for the highest-risk suppliers in the mapped network.
Phase 3: Scale and Integrate (Months 19-36)
- Expand to Full Tier 1 and Key Tier 2: Broaden the program to include all direct suppliers and critical sub-tier suppliers.
- Integrate Systems: Connect the traceability platform with internal ERP and QMS for automated data flow.
- Explore Advanced Tech (Blockchain): For the most complex and critical supply chains, evaluate a blockchain-based solution for a consortium of key partners to share immutable provenance data.
Future Trends: The Evolving Transparency Landscape
Technology and regulation will continue to raise the bar for what constitutes acceptable transparency.
Regulatory and Standardization Drivers
- Digital Product Passports (DPPs): An EU initiative, part of the Ecodesign for Sustainable Products Regulation (ESPR), that will require a digital record of a product’s environmental and material data throughout its lifecycle.
- Harmonized ESG Reporting: Convergence of standards (ISSB, GRI) will demand more granular, auditable supply chain data on Scope 3 emissions and social impact.
- DNA and Isotopic Marking: Forensic techniques to physically “tag” raw materials or intermediates to provide incontrovertible proof of origin, moving beyond paperwork.
AI and Predictive Analytics
- AI for Risk Prediction: Machine learning models that analyze news, satellite imagery, and financial data to predict supplier instability or ethical violations before they occur.
- Automated Document Analysis: AI to scan and verify supplier documents (audits, certificates) for inconsistencies or fraud.
FAQs: Peptide Supply Chain Transparency and Ethical Sourcing
Q: As a small peptide developer, we have limited leverage over our large, global suppliers. How can we effectively demand transparency and ethical data from them?
A: Your leverage is not in size, but in the clarity and unity of your requirements. Start by integrating a robust Supplier Code of Conduct and a simple transparency questionnaire into your standard Quality Agreement. Frame it as an industry-wide shift essential for regulatory compliance and patient trust. You can also reference the requirements of your larger pharma partners (if applicable) to show this is a non-negotiable market demand. For critical sole-source suppliers, emphasize that transparency is a condition for a long-term, strategic partnership.
Often, joining an industry consortium or initiative (like the PSCI) can provide collective leverage and standardized tools that make it easier for suppliers to comply with multiple customers at once.
Q: What is the most critical piece of data to collect from suppliers to begin building a transparent supply chain?
A: The most critical foundational data point is a complete, accurate multi-tier bill of materials (BOM) for your peptide API, provided by your direct manufacturer. This should list not just the major building blocks (e.g., Fmoc-amino acids), but all reagents, solvents, and catalysts, along with their manufacturers (Tier 2). Without this map, you cannot know what to trace. The second most critical item is the Country of Origin for each of those input materials. Starting with this information allows you to conduct a risk assessment and begin targeted engagement with the highest-priority sub-tier suppliers for further verification.
Q: How do blockchain-based traceability systems handle confidential business information (CBI), like synthesis details or exact pricing?
A: Permissioned blockchains are designed precisely for this challenge. Data is shared on a need-to-know basis. While the hash (a unique digital fingerprint) of a transaction is recorded on the immutable ledger for all to verify, the actual transaction details (e.g., the CoA, batch record) are stored “off-chain” in a secure, encrypted database. Access to this detailed data is controlled by smart contracts and permissions.
A supplier can share full batch data with their direct customer but only prove the *existence* of a valid CoA (via the hash) to other parties in the network without revealing the sensitive data. This ensures provenance and integrity are verified while protecting intellectual property and commercial terms.
Core Takeaways
- Transparency is a Strategic Asset: For peptide companies, supply chain visibility is no longer optional; it is a critical capability for managing risk, ensuring quality, and meeting the ethical expectations of regulators, investors, and patients.
- Technology Enables Trust: Digital traceability systems (serialization, IoT, blockchain) create the “digital thread” that provides immutable proof of provenance, custody, and condition, transforming opaque chains into transparent networks.
- Verification is Multi-Dimensional: Ethical sourcing must be verified through a combination of robust due diligence, independent audits, trusted certifications, and the collection of verifiable documentary evidence, not just supplier questionnaires.
- Implementation is a Phased Journey: Start by mapping and assessing the highest-risk suppliers, then digitize core flows, and finally scale and integrate advanced technologies. A pragmatic, stepwise approach ensures sustainable progress.
- Collaboration is Fundamental: True end-to-end transparency cannot be achieved unilaterally. It requires building collaborative partnerships with suppliers based on shared standards, data exchange, and mutual commitment to ethical practices.
Conclusion: Delivering Integrity with Every Dose
The pursuit of supply chain transparency and ethical sourcing in the peptide industry is a profound alignment of commercial necessity with moral responsibility. It moves the definition of quality beyond the certificate of analysis to encompass the entire narrative of the product’s creation. By investing in the technologies and partnerships that illuminate the supply chain, companies do more than mitigate risk—they build a formidable foundation of trust, resilience, and brand integrity that will define the winners in the sustainable economy of the future.
This journey requires expertise, commitment, and partnership. It begins with choosing suppliers who share this vision and possess the operational discipline to support it. Sichuan Pengting Technology Co., Ltd. is committed to being such a partner. As a professional and reliable peptide API supplier, we understand that our responsibility extends beyond delivering a high-purity product. We are investing in the systems and processes that provide our clients with unprecedented supply chain clarity. From detailed sourcing declarations for our raw materials to robust data capture throughout our synthesis and purification processes, we enable the traceability and verification that modern drug developers require.
Partnering with Sichuan Pengting Technology means securing not just a quality API, but also the confidence that comes from a transparent, ethical, and fully accountable supply chain—a critical component in bringing trustworthy therapies to market.
Disclaimer
This article contains information, data, and references that have been sourced from various publicly available resources on the internet. The purpose of this article is to provide educational and informational content. All trademarks, registered trademarks, product names, company names, or logos mentioned within this article are the property of their respective owners. The use of these names and logos is for identification purposes only and does not imply any endorsement or affiliation with the original holders of such marks. The author and publisher have made every effort to ensure the accuracy and reliability of the information provided.
However, no warranty or guarantee is given that the information is correct, complete, or up-to-date. The views expressed in this article are those of the author and do not necessarily reflect the views of any third-party sources cited.




