In the hyper-competitive landscape of the global peptide therapeutics market, projected to exceed $100 billion, success is not merely a function of scientific brilliance but of strategic acumen. With over 150 peptide drugs in clinical development and a surge of investment from both large pharma and agile biotechs, companies can no longer afford to operate with an inward-looking R&D focus. A reactive stance to competitor moves, market shifts, and technological disruptions is a direct path to irrelevance. Proactive, systematic competitive intelligence, crystallized through frameworks like the SWOT (Strengths, Weaknesses, Opportunities, Threats) analysis, is the essential compass for navigation. This article provides a comprehensive, actionable guide to conducting a rigorous peptide-specific competitive analysis, transforming raw data into a dynamic SWOT assessment, and, most critically, building a strategic response plan that turns insights into sustainable competitive advantage.
From Data to Direction: The Imperative of Strategic Competitive Analysis
In the peptide space, where development timelines are long and capital requirements are high, strategic missteps are extraordinarily costly.
The High Cost of Strategic Myopia
Operating without a clear view of the competitive landscape leads to predictable failures:
- Duplicative R&D Investment: Wasting resources developing a “me-too” peptide in a crowded target space where a competitor is 18 months ahead.
- Suboptimal Resource Allocation: Investing in a manufacturing technology that is becoming obsolete due to a competitor’s breakthrough in continuous synthesis.
- Missed Partnership & Exit Opportunities: Failing to identify potential acquirers or complementary technology partners because their strategic needs are not understood.
- Pricing and Market Access Failure: Launching a therapy with an unsustainable price because the value proposition versus established and pipeline competitors was poorly defined.
Competitive Analysis vs. Competitive Intelligence
It’s crucial to distinguish between the activity and the outcome:
- Competitive Analysis: The systematic process of gathering, analyzing, and interpreting information about competitors, the market, and the broader environment.
- Competitive Intelligence (CI): The actionable insights and strategic implications derived from the analysis. SWOT is the classic framework for synthesizing CI.
“In the peptide race, knowing your own science is table stakes. Knowing your competitors’ science, their manufacturing capabilities, their regulatory strategy, and their financial runway is what separates the winners from the also-rans. A dynamic SWOT isn’t a poster for the wall; it’s the living, breathing dashboard for your entire corporate strategy.” — Marcus Vance, Chief Strategy Officer, Global Bio-Strategy Partners.
Building the Intelligence Foundation: Data Collection for Peptide Competitors
Effective analysis is built on a structured approach to gathering diverse data streams.
Identifying and Categorizing Competitors
Not all competitors are equal. Categorize to focus effort:
| Competitor Type | Definition (Peptide Context) | Key Data Sources |
|---|---|---|
| Direct Competitors | Companies developing peptides for the same primary indication, with a similar mechanism of action (MoA) and patient population. | Clinical trial registries (ClinicalTrials.gov), competitor pipelines, scientific publications, conference presentations. |
| Indirect Competitors | Companies with different modalities (e.g., monoclonal antibody, small molecule, gene therapy) targeting the same disease pathway or endpoint. | Medical literature, analyst reports, therapy class reviews. |
| Platform/Technology Competitors | Companies developing competing enabling technologies (e.g., novel delivery systems, conjugation chemistries, peptide discovery platforms). | Patent databases, technology review journals, investor presentations from platform biotechs. |
| Potential Future Competitors | Large pharma with relevant expertise but no declared peptide program, or academic labs with groundbreaking early-stage research. | Company strategy announcements, university tech transfer offices, grant databases. |
The Intelligence Toolkit: Key Data Dimensions
For each key competitor, profile them across multiple dimensions:
- Product & Pipeline: Stage of development, clinical data (efficacy, safety), formulation, dosing, target product profile (TPP).
- Technology & IP: Core patents (composition, method of use, formulation), freedom-to-operate landscape, unique technology platforms.
- Commercial & Market: Pricing strategy, market access plans, sales force, key opinion leader (KOL) affiliations, promotional materials.
- Manufacturing & Supply: API source (internal vs. CDMO), scale-up capabilities, cost of goods (COGS) estimates, any publicized supply issues.
- Corporate & Financial: Financial health, funding runway, partnership history, M&A appetite, corporate culture, leadership team background.
The SWOT Framework: A Dynamic Lens for Peptide Strategy
SWOT organizes intelligence into four quadrants, providing a balanced view of internal and external factors.
Conducting the SWOT Analysis: A Practical Guide
Each quadrant asks a specific set of questions relevant to a peptide company:
| SWOT Quadrant | Core Question | Peptide-Specific Examples |
|---|---|---|
| Strengths (Internal, Positive) | What do we do better than anyone else? What unique resources do we control? | Proprietary long-acting delivery technology; world-class peptide medicinal chemistry team; exclusive in-licensed cyclic peptide library; validated, scalable GMP manufacturing process. |
| Weaknesses (Internal, Negative) | Where are we vulnerable? What do our competitors do better? | Limited cash runway (< 18 months); reliance on a single CDMO for API; lack of in-house commercial team; clinical program lags behind the competitor’s Phase 3 asset. |
| Opportunities (External, Positive) | What favorable market trends can we exploit? Where are competitors failing? | Growing investor interest in metabolic disease peptides; competitor’s drug showed liver toxicity signal; new regulatory pathway for rare disease peptides; untapped Asian market for a generic peptide. |
| Threats (External, Negative) | What challenges could harm us? What are competitors doing that is a threat? | New, stringent pharmacopoeial requirement for peptide aggregates; key raw material (Fmoc-amino acid) shortage; competitor’s expected FDA approval in 9 months; potential for price erosion from biosimilars. |
Avoiding Common SWOT Pitfalls
- Vagueness: “Strong science” is not a strength. “Our GLP-1/GIP dual agonist peptide has shown superior HbA1c reduction in Phase 2 versus semaglutide” is a strength.
- Static Analysis: A SWOT must be a living document, updated quarterly with new intelligence.
- Ignoring the “So What?”: Listing items without connecting them is pointless. Every item should feed into strategic planning.
From Analysis to Action: Strategic Response Planning
The true value of SWOT is realized in the strategic choices it informs. This is done through a TOWS matrix, which matches internal and external factors.
The TOWS Matrix: Generating Strategic Options
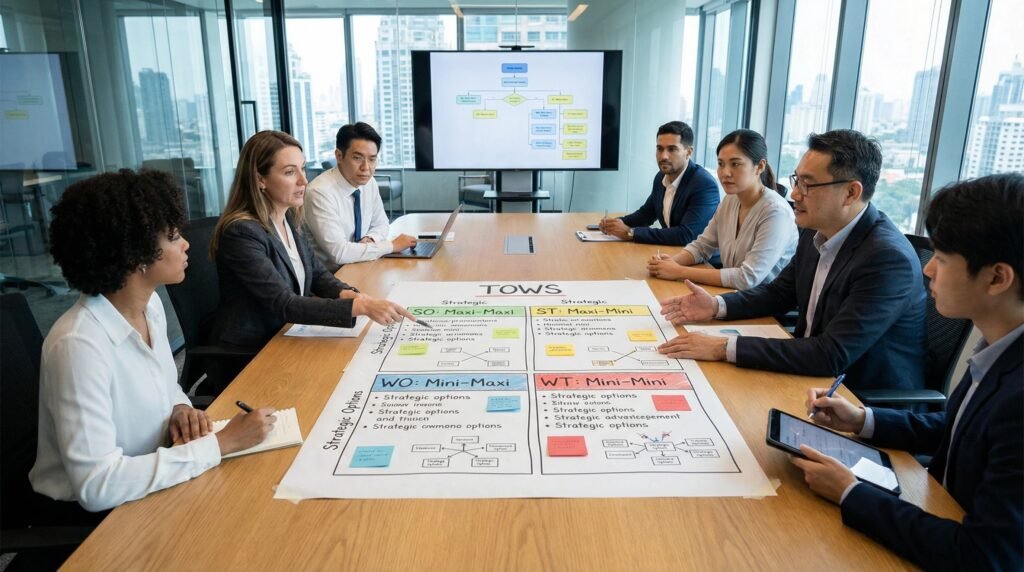
TOWS systematically explores how to leverage the SWOT analysis:
| Strategy Type | Logic | Generated Strategic Option (Example) |
|---|---|---|
| SO Strategies (Maxi-Maxi) Use Strengths to exploit Opportunities. | Leverage what you’re good at to seize an external chance. | Strength: Best-in-class oral peptide platform. Opportunity: High unmet need for oral GLP-1s. Strategy: Fast-track development of oral GLP-1 candidate and seek Breakthrough Therapy designation. |
| ST Strategies (Maxi-Mini) Use Strengths to mitigate Threats. | Use your advantages to defend against external dangers. | Strength: Strong patent estate on manufacturing process. Threat: Competitor’s pending patent on a similar process. Strategy: File an IPR (Inter Partes Review) against the competitor’s patent to protect market space. |
| WO Strategies (Mini-Maxi) Use Opportunities to overcome Weaknesses. | Use external chances to fix internal problems. | Weakness: No commercial infrastructure. Opportunity: Large pharma X is seeking to in-license late-stage metabolic assets. Strategy: Pursue a licensing deal with Pharma X for ex-North America rights, using their capital and commercial muscle. |
| WT Strategies (Mini-Mini) Minimize Weaknesses to avoid Threats. | Fix internal flaws to survive external dangers. | Weakness: Sole-source API supplier. Threat: Geopolitical risk in supplier’s region. Strategy: Initiate a dual-sourcing strategy immediately by qualifying a second API manufacturer to build supply chain resilience. |
Prioritizing and Executing the Strategic Plan
- Prioritize Initiatives: Use an impact/effort matrix. Focus on high-impact, feasible strategies first (e.g., WT strategies are often urgent).
- Define Clear Objectives & KPIs: Turn “pursue a licensing deal” into “Secure a term sheet with a top-20 pharma for ex-US rights by Q4, with minimum $50M upfront.”
- Assign Ownership & Resources: Each strategic initiative must have a named owner, a budget, and a timeline.
- Integrate with Corporate Planning: The output of the SWOT/TOWS exercise should feed directly into the annual strategic plan, budget, and board updates.
Future Trends: AI-Enhanced Competitive Intelligence
The field of competitive analysis is being transformed by technology.
- AI-Powered Data Aggregation: Machine learning models that continuously scan clinical databases, patents, financial news, and social media to identify competitor signals and trends automatically.
- Predictive Analytics: Using historical data on competitor behavior and clinical success rates to model likely future moves (e.g., probability of a competitor entering Phase 3, likelihood of an acquisition).
- Network Analysis: Mapping the relationships between KOLs, academic institutions, and competitors to identify influence patterns and partnership opportunities.
- Real-Time Dashboards: Moving from static quarterly reports to live competitive intelligence dashboards accessible to key decision-makers.
FAQs: Peptide Competitive Analysis and SWOT
Q: As a small virtual biotech with one lead peptide asset, how can we possibly compete with the intelligence resources of a large pharmaceutical company?
A: Your advantage is focus and agility. While a large pharma scans broadly, you can go deep on the 5-10 entities that truly matter to your asset’s future. Use focused, often free tools: set up Google Alerts for key competitors and KOLs, monitor Clinical Trials.gov weekly, and thoroughly analyze competitor SEC filings and investor presentations. Your small size allows for rapid decision-making based on intelligence. Furthermore, you can outsource specific intelligence tasks to specialized consultants. Your deep, focused knowledge in your niche can often uncover insights that a large, decentralized pharma team might miss.
Q: How often should we formally update our SWOT analysis, and who should be involved in the process?
A: A formal, cross-functional review should occur at least quarterly, with a lighter monthly check-in on key intelligence updates. The core team should include heads of Strategy, R&D, Business Development, Commercial (if applicable), and Regulatory Affairs. Input should also be sought from key scientists and the financial team. The process should be facilitated to ensure it is a strategic dialogue, not just a data-dumping session. The output is a revised set of strategic priorities, not just an updated document.
Q: In the TOWS matrix, how do we decide between an “SO” growth strategy and a necessary “WT” defensive strategy when resources are limited?
A: This is the essence of strategic choice. A useful framework is to assess strategic risk vs. survival risk. “WT” strategies (like fixing a critical supply chain weakness) often address existential survival risks—if not done, the company may fail. These must be prioritized first, even if they are not “sexy.” “SO” strategies are about growth and maximizing upside. The allocation should reflect the company’s stage: a pre-revenue biotech may need to spend 70% of its resources on “WT/WO” strategies to de-risk and survive, while a commercial-stage company can invest more in “SO” growth. A balanced portfolio that addresses critical vulnerabilities while positioning for future growth is ideal.
Core Takeaways
- Competitive Analysis is Non-Negotiable: In the crowded peptide market, systematic intelligence gathering and analysis are foundational to survival and success, not optional activities.
- SWOT Provides the Strategic Lens: A well-executed SWOT analysis forces an honest assessment of internal capabilities and external market dynamics, creating a shared reality for the leadership team.
- The TOWS Matrix is the Action Engine: The true value is unlocked by using the TOWS framework to generate specific, actionable strategic options (SO, ST, WO, WT) from the SWOT data.
- Execution is Everything: Insights must be translated into a prioritized plan with clear owners, resources, and KPIs, integrated into the core business planning cycle.
- It’s a Continuous Process: Competitive landscapes are fluid. Intelligence must be gathered continuously, and the SWOT/strategy must be revisited and revised regularly, not treated as an annual exercise.
Conclusion: Building an Antifragile Peptide Enterprise Through Strategic Foresight
Mastering competitive analysis and strategic response planning is what separates the peptide companies that merely survive from those that define the future of the industry. By institutionalizing a process of continuous intelligence gathering, rigorous SWOT assessment, and disciplined strategic choice via TOWS, organizations can move from a position of reaction to one of proactive creation. This capability builds an “antifragile” enterprise—one that does not just withstand market shocks and competitive threats but uses them as information to adapt and strengthen its position.
This strategic discipline extends to every partnership in the value chain. A company’s resilience is linked to the reliability and strategic alignment of its core suppliers. Sichuan Pengting Technology Co., Ltd. understands this deeply. As a professional and reliable peptide API supplier, we recognize that our role extends beyond delivering a quality ingredient. We act as a strategic partner in our clients’ competitive journeys. Our manufacturing reliability and expertise directly strengthen our clients’ internal Strengths (robust supply chain) and mitigate key Weaknesses (supply risk). Our investment in scalable, efficient processes helps clients capitalize on Opportunities (market demand) and defend against Threats (cost pressure). By choosing a partner like Sichuan Pengting Technology, peptide innovators secure more than an API; they gain a strategic ally whose capabilities and stability directly contribute to a more formidable and responsive competitive position in the global marketplace.
Disclaimer
This article contains information, data, and references that have been sourced from various publicly available resources on the internet. The purpose of this article is to provide educational and informational content. All trademarks, registered trademarks, product names, company names, or logos mentioned within this article are the property of their respective owners. The use of these names and logos is for identification purposes only and does not imply any endorsement or affiliation with the original holders of such marks. The author and publisher have made every effort to ensure the accuracy and reliability of the information provided. However, no warranty or guarantee is given that the information is correct, complete, or up-to-date. The views expressed in this article are those of the author and do not necessarily reflect the views of any third-party sources cited.