The global peptide therapeutics market, projected to exceed $75 billion by 2028, is revolutionizing treatment paradigms across oncology, metabolic disease, and immunology. This rapid expansion brings intensified regulatory scrutiny, particularly concerning the control of potentially mutagenic impurities that could pose a carcinogenic risk to patients. While the ICH M7 guideline, “Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals,” has provided a robust framework for small molecules, its application to peptide APIs presents unique and often misunderstood scientific and regulatory challenges. Synthetic peptide manufacturing, with its complex sequences, specialized protecting groups, and multi-step processes involving cleavage reagents, can introduce impurities not typically found in traditional organic synthesis.
This comprehensive guide demystifies the application of ICH M7 principles to peptide drug substances, detailing modern, risk-based assessment strategies, advanced analytical control methods, and a clear roadmap for ensuring patient safety and global regulatory compliance.
The Uncompromising Mandate: Controlling Mutagenic Impurities in Therapeutics
The presence of genotoxic impurities, even at trace levels, is unacceptable due to their potential to cause DNA damage and initiate carcinogenesis. This makes their control a non-negotiable cornerstone of drug safety.
The Genesis and Purpose of ICH M7
ICH M7 was established to provide a standardized, internationally harmonized framework for:
- Identifying potential mutagenic impurities in drug substances and products.
- Controlling these impurities to levels (Threshold of Toxicological Concern, TTC) that present a negligible risk (theoretical excess cancer risk of <1 in 100,000 over a lifetime).
- Providing a Practical Framework that balances patient safety with the feasibility of impurity control during manufacturing.
Why Peptides Present a Distinct Challenge
Peptide synthesis and degradation pathways introduce unique impurity profiles:
- Complex Degradation Products: Peptides can form unique degradation impurities (e.g., diketopiperazines, deamidation products, oxidation derivatives) whose genotoxic potential is not always predictable from the parent structure.
- Specialized Reagents and By-products: The synthesis employs reagents like strong acids (TFA, HF for cleavage), carbodiimides for coupling, and scavengers, which can leave residues or form reactive by-products.
- Sequence-Dependent Impurities: Deletion sequences, truncated peptides, and epimerized amino acids are peptide-specific impurities whose toxicological profiles require assessment.
- Polymeric and Aggregate Impurities: The potential for peptide aggregation, while primarily a product quality concern, may also require evaluation of novel chemical entities formed during the process.
“Applying ICH M7 to peptides cannot be a simple checkbox exercise. It demands a deep understanding of peptide chemistry to ask the right questions: Which reagent by-product could be mutagenic? Could a novel dimer formed during stress have structural alerts? A proactive, chemistry-driven assessment is the only way to build a truly defensible control strategy for a modern peptide API.” — Dr. Kenji Tanaka, Head of Toxicology and Impurity Strategy, Global CMC Consultants.
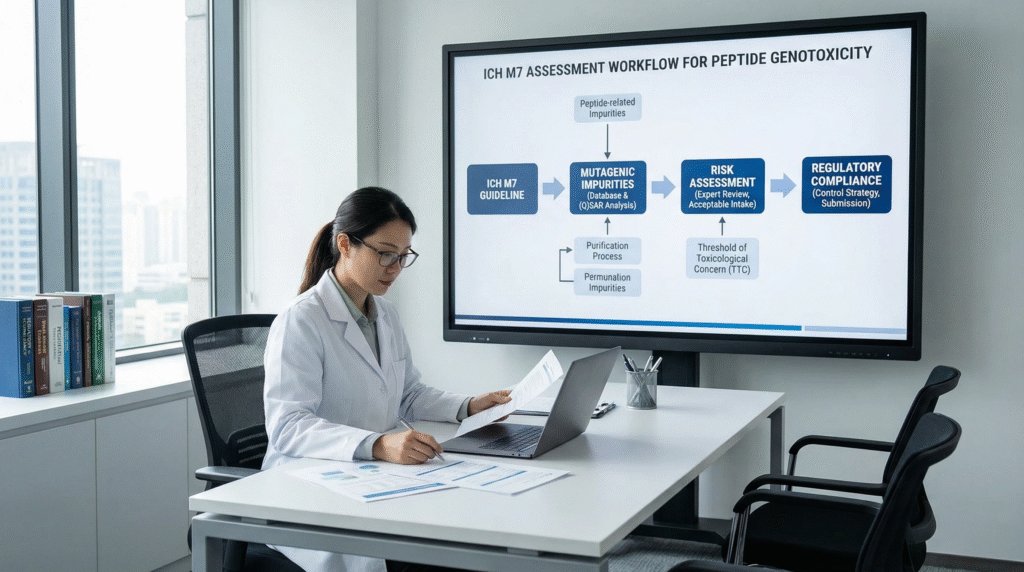
Decoding the ICH M7 Framework: A Tiered Approach to Assessment
ICH M7 outlines a structured, risk-based workflow for impurity assessment, which forms the backbone of any peptide impurity control strategy.
The Five-Class Categorization System
Every impurity must be classified to determine the required level of control:
| Class | Definition | Required Action for Peptide Impurities |
|---|---|---|
| 1 | Known mutagenic carcinogens. | Control at or below compound-specific acceptable limits. Requires stringent, validated analytical methods. |
| 2 | Known mutagens with unknown carcinogenic potential (positive Ames test). | Control at or below acceptable limits (appropriate TTC). |
| 3 | Alerting structure, unrelated to the drug substance; no mutagenicity data. | Conduct bacterial mutagenicity assay (Ames test). If negative, treat as Class 5. If positive, treat as Class 2. |
| 4 | Alerting structure, same alert in drug substance which is tested and non-mutagenic. | Can be treated as Class 5. Requires expert review to confirm the alert is adequately “purged” of mutagenic potential in the specific impurity. |
| 5 | No structural alerts for mutagenicity or sufficient data to negate the alert. | Control per ICH Q3A/Q3B guidelines for ordinary impurities. |
The Critical Role of (Q)SAR Predictions
In silico toxicology prediction is the first and primary screening tool:
- Dual Methodology: ICH M7 requires the use of two complementary (Q)SAR methodologies: one rule-based (e.g., Derek Nexus) and one statistical-based (e.g., Sarah, CASE Ultra).
- Assessing “Structural Alerts”: The software identifies molecular substructures associated with DNA reactivity. For peptides, this includes assessing not just the final molecule, but all potential impurities arising from reagents, degradation, and side reactions.
- Expert Review is Paramount: (Q)SAR outputs are not final answers. A trained toxicologist or chemist must interpret the results, especially for large molecules like peptides where alerts may be masked or irrelevant in the broader context.
Applying the Framework to Peptide API: A Step-by-Step Strategy

A proactive, phase-appropriate strategy is essential for efficient and compliant peptide development.
Step 1: Early Risk Assessment (Preclinical/Phase I)
- Map the Synthetic Route: Identify all starting materials, reagents, solvents, catalysts, and potential degradants.
- Conduct (Q)SAR Screening: Perform in silico assessment on the drug substance and a comprehensive list of theoretical impurities from the route and degradation pathways.
- Prioritize Impurities for Control: Focus analytical method development on impurities with structural alerts (Class 3) that are likely to be present based on the route.
- Define Initial Control Strategy: Set conservative limits and specify controls in the drug substance specification for any high-risk impurities.
Step 2: Analytical Control and Method Development
Detecting and quantifying impurities at the TTC level (often 1-5 ppm) requires advanced techniques:
| Analytical Challenge | Recommended Techniques for Peptides |
|---|---|
| Detection of Non-Peptide Mutagenic Impurities (e.g., alkyl sulfonates, hydrazines) | LC-MS/MS with sensitive, targeted methods; Derivatization GC-MS for volatile impurities. |
| Detection of Peptide-Related Impurities with Alerts (e.g., certain deletion sequences) | High-Resolution LC-MS (Q-TOF, Orbitrap) for accurate mass identification; Peptide mapping with advanced detection. |
| Routine Release and Stability Testing | Validated, stability-indicating HPLC/UPLC methods with sufficient sensitivity, often with MS confirmation for identified mutagens. |
Step 3: The Control Strategy: Purge Arguments and Specification Setting
For impurities predicted to be mutagenic, control can be achieved through testing, process understanding, or both.
- Test for Presence: The direct approach. Implement a validated analytical procedure to control the impurity at or below its acceptable limit in the drug substance.
- Purge by Process Understanding: A powerful alternative for peptide synthesis. This involves:
- Demonstrating that the impurity is removed (purged) during downstream processing (e.g., cleavage, precipitation, washes, chromatography) to a level below the acceptable limit.
- Providing scientific rationale and, ideally, spiking study data to show the impurity’s fate during purification.
- This is often applicable to mutagenic reagents and their by-products used in early synthetic steps.
- Setting Appropriate Specifications: The control strategy must be clearly defined in the drug substance specification, referencing either a test with a defined limit or a justified purge argument.
Common Pitfalls and Best Practices for Peptide Developers
Successfully navigating ICH M7 for peptides requires awareness of common mistakes.
Pitfall 1: Overlooking Degradation Products
Assuming only process-related impurities need assessment. Stress studies (hydrolytic, oxidative, thermal) must be performed, and major degradation products must undergo (Q)SAR assessment.
Pitfall 2: Misinterpreting (Q)SAR Alerts for Large Molecules
An alert on a single amino acid within a 40-mer peptide may not render the entire molecule mutagenic if the reactive moiety is sterically inaccessible. Expert review is critical to avoid unnecessary testing.
Pitfall 3: Inadequate Purging Data
A weak purge argument without supporting data (e.g., solubility, reactivity, chromatographic behavior) is a common regulatory deficiency. Invest in small-scale spiking studies to generate robust data.
Best Practice: Integrated, Cross-Functional Team
Genotoxicity assessment is not solely a toxicology function. It requires close collaboration between peptide chemists, analytical scientists, process engineers, and regulatory affairs to be effective.
Future Trends: Advanced Tools and Evolving Expectations
The science of impurity assessment continues to advance.
- New Approach Methodologies (NAMs): Increased use of in vitro and in chemico assays beyond Ames to better understand the mechanism of action for flagged impurities.
- AI-Enhanced (Q)SAR and Prediction: Machine learning models trained on larger datasets, potentially offering better predictions for complex peptide-related structures.
- Increased Regulatory Focus on Nitrosamines: The nitrosamine saga has heightened awareness of mutagenic impurities globally. Peptide processes using amine-containing reagents in the presence of nitrosating agents must be rigorously evaluated.
FAQs: Peptide Genotoxicity Assessment and ICH M7
Q: Do all amino acids or protecting groups in my peptide synthesis need to be screened for mutagenicity?
A: Yes, a comprehensive assessment is required. This includes the specific Fmoc/Boc-amino acids used, the coupling reagents (e.g., HATU, DIC), cleavage cocktail components (TFA, water, scavengers like triisopropylsilane), and any other reagents or solvents. The assessment focuses not just on the reagents themselves, but on potential reactive by-products that could form and carry through (e.g., hydrazine from a carbodiimide reagent). A qualified supplier providing reagents with appropriate controls is essential, but the sponsor (drug applicant) retains ultimate responsibility.
Q: How do we handle a peptide-specific impurity (like a deletion sequence) that triggers a (Q)SAR alert? Does it require an Ames test?
A: A peptide-specific impurity with a structural alert (Class 3) would typically trigger the requirement for an Ames test to resolve its mutagenic potential, unless a strong scientific argument can be made for classification into Class 4 or 5. The argument for Class 4 would require demonstrating that the alerting structure is present in the parent drug substance (which is non-mutagenic) and that the impurity’s formation does not create a new, reactive context for that alert. This requires expert toxicology judgment.
Given the cost and time of synthesizing the impurity for testing, a robust purge argument showing it is controlled below the TTC may sometimes be a more feasible control strategy.
Q: What is the role of the API manufacturer versus the drug product sponsor in ICH M7 compliance?
A: The responsibility is shared but distinct. The API manufacturer (e.g., Sichuan Pengting Technology Co., Ltd.) is responsible for the genotoxicity assessment and control of impurities introduced during the synthesis of the drug substance. This includes providing a detailed impurity assessment report, supporting (Q)SAR data, analytical validation for controlled mutagens, and robust purge arguments. The drug product sponsor (the pharmaceutical company) holds the regulatory submission and ultimate responsibility.
They must audit the API manufacturer’s system, review and integrate the assessment into the overall drug product dossier, and assess any additional risk from drug product formulation or storage. A transparent, collaborative relationship is key to a successful submission.
Core Takeaways
- Proactive, Not Reactive: ICH M7 assessment must be integrated into early peptide process development, not added as a last-minute compliance activity before submission.
- Chemistry-Driven Assessment: A deep understanding of peptide synthesis and degradation chemistry is the most critical factor in identifying potential mutagenic impurities that (Q)SAR might miss.
- Control via Testing or Purge: A robust control strategy can be based on sensitive analytical testing or on a scientifically justified process purge argument, supported by data.
- Expertise is Non-Negotiable: Interpreting (Q)SAR results for complex peptides and building defendable purge arguments requires specialized toxicological and chemical expertise.
- Collaborative Partnership is Essential: Success depends on seamless collaboration between the drug sponsor and an API manufacturer equipped with the right systems, scientific rigor, and regulatory understanding.
Conclusion: Building a Foundation of Safety for Peptide Innovation
Navigating the requirements of ICH M7 for peptide APIs is a sophisticated exercise in proactive risk management, demanding a blend of advanced analytical science, computational toxicology, and deep peptide chemistry expertise. In an era of heightened regulatory scrutiny around impurity control, a robust and defensible genotoxicity assessment strategy is not merely a regulatory hurdle—it is a fundamental component of product quality, patient safety, and sustainable commercial success. By adopting a structured, science-based approach from the earliest stages of development, peptide innovators can confidently ensure their therapies meet the highest global safety standards.
This complex journey is significantly de-risked by partnering with an API manufacturer that embodies these principles. Sichuan Pengting Technology Co., Ltd. integrates ICH M7 compliance into the very fabric of its peptide development and manufacturing services. As a professional and reliable peptide API supplier, we provide our clients with more than just a molecule; we deliver a comprehensive, data-backed package that includes rigorous (Q)SAR assessments, scientifically sound purge justifications, and validated analytical control methods for mutagenic impurities. Our team of experts works transparently with clients to design synthetic routes that minimize genotoxic risk and to build the compelling evidence needed for successful global regulatory submissions.
Choosing a partner like Sichuan Pengting Technology ensures that your innovative peptide therapeutic is built on an uncompromising foundation of quality and safety.
Disclaimer
This article contains information, data, and references that have been sourced from various publicly available resources on the internet. The purpose of this article is to provide educational and informational content. All trademarks, registered trademarks, product names, company names, or logos mentioned within this article are the property of their respective owners. The use of these names and logos is for identification purposes only and does not imply any endorsement or affiliation with the original holders of such marks. The author and publisher have made every effort to ensure the accuracy and reliability of the information provided.
However, no warranty or guarantee is given that the information is correct, complete, or up-to-date. The views expressed in this article are those of the author and do not necessarily reflect the views of any third-party sources cited.




